If everything goes to plan, Cuba could be the first Latin American country to develop and manufacture its own vaccine against COVID-19.
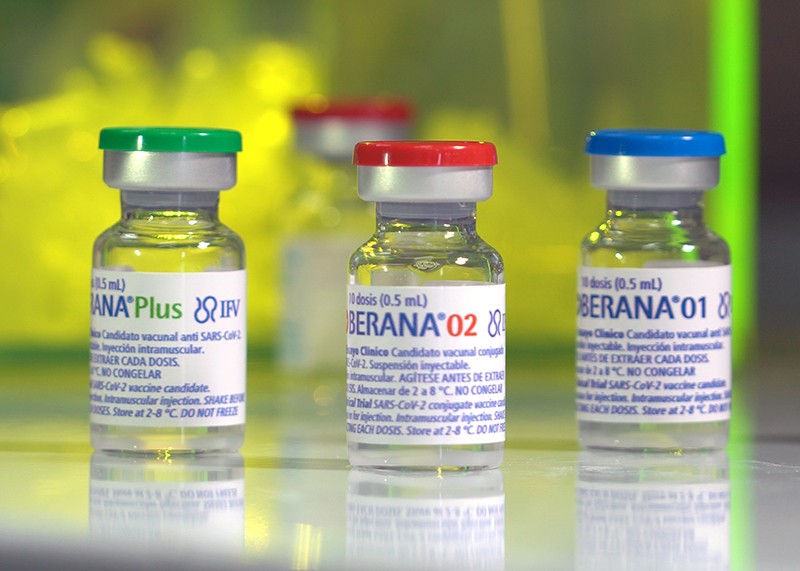
Vicente Vérez Bencomo, director-general of the state-owned Finlay Institute of Vaccines in Havana, where one of Cuba’s most advanced vaccine candidates was created, thinks the chances are good. The candidate, called Soberana 02, entered phase III trials in people in March. It’s one of the country’s two homegrown vaccines — the other is called Abdala — to make it this far.
And not a moment too soon. Although Cuba had few infections for most of 2020, COVID-19 cases began to rise in the 11-million-person island nation after it reopened its borders to tourism last November. Infections peaked on 24 April, with nearly 5,800 active cases.
Cuba is one of the last-remaining communist countries in the world, and has endured decades of trade embargoes imposed by the United States, cutting off its access to essential supplies. Vérez Bencomo says it is this history that has given the Cuban people an independent streak, spurring them to create their own COVID-19 jab rather than joining the international COVAX initiative, which aims to deliver vaccines fairly across all countries.
Even Soberana 02 has an independent streak, working differently from other vaccines in play. The jab is a ‘conjugate’ vaccine, one that links a weaker antigen with a stronger one to ensure a vigorous immune response. To make Soberana 02, Finlay scientists coupled fragments of the coronavirus spike protein to a deactivated form of tetanus toxin, a powerful antigen that can boost production of immune cells and antibodies1.
Nature spoke to Vérez Bencomo about Soberana 02, Cuba’s decision to go solo, and the difficulties of doing science under a forceful economic blockade.
When did Finlay join the COVID-19 vaccine race?
Around May 2020, there was a major call from our president, Miguel Díaz-Canel, for anybody who could develop a vaccine against the coronavirus to do so. It was very important for us. We foresaw that when vaccines were ready [in other parts of the world], it would take a long time for them to reach countries like ours.
Of course, by joining the race, we had to abandon other projects. We stopped a clinical trial with a pneumococcus vaccine. We had a very innovative whooping-cough vaccine that we also disrupted. It was not possible to continue doing anything else.
How many different vaccines is Finlay testing right now?
We have three vaccines in the Soberana series. We’re testing Soberana 02 with 44,000 people, some of whom are getting a placebo, in a phase III trial. And because of the urgency, we’re also conducting another effectiveness trial in 75,000 people without placebo. Because not everyone gets vaccinated at the same time, the people waiting for their shot will serve as a control group.
Ethically, it is too late to launch any new placebo studies in Cuba because COVID-19 cases are ramping up. So to test Soberana 01 [a non-conjugate vaccine containing pairs of spike-protein fragments, as well as components from the outer shells of meningococcal bacteria to boost the immune response], we’re designing a protocol to compare it with Soberana 02, instead of using a placebo. We’re awaiting the approval from Cuba’s national regulatory authority to begin the phase II trial.
We also have a trial with 450 convalescent individuals, who recovered from COVID-19 or were asymptomatic, in which we’re testing Soberana Plus, a booster dose that contains spike-protein fragments. This vaccine is designed to re-stimulate the initial immunity people got from a previous infection.
How do the Soberana 02 results look so far?
What I can reveal is that during the previous trial phases, two doses of Soberana 02 generated an antibody response in about 80% of vaccinated people. But applying a third, booster dose of Soberana Plus raised that percentage to 100%, all of them with neutralizing antibodies that can block the virus from entering cells.
To what extent will that protect people from death? I’m sure it’ll protect them. To what extent will that protect people from severe disease? It’s part of what the efficacy [phase III] trial has to prove, but we think it will. We think that we should have results ready to publish by June.
Talk about the vaccine line’s name, Soberana, which translates as ‘sovereign’.
In a meeting we had with the president, he told us that we needed to have sovereignty over our vaccines.
After we announced the first Soberana trial, people liked the name so much that it was impossible to change it. This was taken with such pride in Cuba that we didn’t have any other choice but to call the vaccine Soberana. People really trust what we do. We always have three times as many people lined up to participate in clinical trials as we need.
Cuba plans to inoculate all its citizens with its own vaccines. Will it have the resources to do that?
We’re speeding up production so that when the Soberana 02 studies are done, we can get authorization for emergency use. We hope this doesn’t take too much time, because we have a very high incidence of COVID in Cuba right now, especially in Havana.
In the face of this emergency, we are reorganizing our manufacturing capacities. We think that sometime this year, we should be able to produce around ten million doses each month.
We have a lot of demand for vaccines right now — much more than what we could supply. So we are looking for serious commitments [to supply jabs abroad] with advanced payments that will allow us to invest the resources we do not have in production.
Why go it alone to develop vaccines instead of joining COVAX?
This is a complex question. There are international initiatives that I respect tremendously. That I respect them is one thing — whether I believe in them is another.
We wanted to solely rely on our own capacities to vaccinate our population, not on other people’s decisions. And life is proving us right. What we’re seeing across the world is that vaccine supplies are being hoarded by rich countries.
How did Cuba find the resources to make its own COVID-19 vaccines?
We’re a very poor country. I can assure you that not one penny of the money used to make medicines or buy food — which are both scarce at the moment — has been diverted for the manufacturing of COVID vaccines.
It’s all been a great individual effort from each of the institutions that are working on this. We’ve all taken the resources we had for other projects and put them into this. And we’ve had to be creative about it. Our scientists are used to doing a lot with very little.
In what ways has the US trade embargo affected vaccine development?
In many ways. We have an American blockade that is not euphemistic at all — it is very real.
Companies that have been selling us materials for 60 years, under former US president Donald Trump’s administration, they got scared and told us, “Sorry, we cannot continue cooperating with you because we are afraid that we will lose our trade with North America.”
It’s very difficult. But we Cubans don’t let ourselves get beaten. We are used to fighting against all odds.
"can" - Google News
April 29, 2021 at 10:19PM
https://ift.tt/3e2197u
Can Cuba beat COVID with its homegrown vaccines? - Nature.com
"can" - Google News
https://ift.tt/2NE2i6G
https://ift.tt/3d3vX4n
Bagikan Berita Ini


















0 Response to "Can Cuba beat COVID with its homegrown vaccines? - Nature.com"
Post a Comment